Transitioning to a strategic sourcing approach.
 The sourcing and supply of commercial drug products – whether it be comparators, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries, and/or consumables for a global clinical trial – is complicated. It can often be a series of challenging tasks that can expose a sponsor company to numerous operational, regulatory and financial risks. And with clinical trials evolving from the more traditional small molecule-high volumes treating broad disease states to large molecule-lower volumes focused on targeted treatments, to now moving more towards gene therapies, one-dose to a cure, makes every aspect of clinical development that much more difficult. A multitude of intricacies often requires a more sophisticated, calculated approach – one that a sourcing specialist like Client-Pharma routinely handles for its clients each and every day. This article will examine a number of commercial drug procurement and supply elements that, when included as part of a sourcing strategy, can effectively reduce a trial sponsor’s exposure to risk.
The sourcing and supply of commercial drug products – whether it be comparators, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries, and/or consumables for a global clinical trial – is complicated. It can often be a series of challenging tasks that can expose a sponsor company to numerous operational, regulatory and financial risks. And with clinical trials evolving from the more traditional small molecule-high volumes treating broad disease states to large molecule-lower volumes focused on targeted treatments, to now moving more towards gene therapies, one-dose to a cure, makes every aspect of clinical development that much more difficult. A multitude of intricacies often requires a more sophisticated, calculated approach – one that a sourcing specialist like Client-Pharma routinely handles for its clients each and every day. This article will examine a number of commercial drug procurement and supply elements that, when included as part of a sourcing strategy, can effectively reduce a trial sponsor’s exposure to risk.
A Strategic Approach
The process of sourcing and supplying commercial drugs for global clinical trials can involve numerous pitfalls for trial sponsors. Examples include an inability to obtain the necessary pedigree or product documentation for study drugs, a lack of supply chain security, with the possible introduction of counterfeit products, and any delays with resupplies throughout the course of the trial.
These are just some of the problems that can lead to significant operational, regulatory, and financial setbacks for the trial sponsor. As such, biopharmaceutical companies must have a comprehensive view of what is involved with their selected supply partners in order to devise a customized, strategic approach which best supports their programs.
In order to minimize this risk of disruption to clinical trials (and the resulting financial implications from potential delays), a commercial drug sourcing and supply strategy should include two key elements:
- A thorough understanding of global regulation governing the sourcing and shipping of the pharmaceutical product(s) concerned
- Ongoing and proactive demand planning that can rapidly accommodate changes in the quantity of commercial drug needed (i.e. in light of fluctuating patient enrollment or unexpected changes to a trial)
Exposure to operational, regulatory, and financial risks can increase if commercial drug sourcing is approached as a series of transactional, commoditized events — i.e. buying a drug from a wholesaler as needed — rather than a process based on a well-thought-out and co-developed strategy. In situations where sourcing becomes an afterthought amidst all other trial requirements, a transactional approach can jeopardize the progress of a study. This is precisely why it is imperative to engage with a specialist, like Client-Pharma, who genuinely understands the importance of involving supply functions early on in the initial planning phases of a clinical trial, such that they can proactively identify any supply pitfalls (i.e. sponsors looking for product from or for particular countries, when there may be limitations or constraints in that region or examining the possibility of better alternatives and strategies, etc.).
In a transactional situation where the sponsor’s clinical supply or procurement teams are faced with sourcing commercial drugs/products (comparators, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries and/or consumable items) at short notice, then a supplier must be quickly identified and vetted – a process that can be challenging in a compressed timeframe. Not a position in which any sponsor wants to be.
If the transactional approach is the only route available for short notice supplies, one of the major risks it presents is that there can be great variability in supply throughout the course of a trial. Inexperienced or non-specialist suppliers may not be able to provide single lots with long expiration dates or the correct documentation – resulting in the purchasing company having to buy the commercial drug in multiple lots, possibly reducing clinical consistency, creating extra packaging and labeling runs, posing a significant challenge should there be a recall – which are all scenarios resulting in increased costs and timelines.
In addition, non-specialist suppliers will not be well-versed in the range of regulations governing use of commercial drugs and the logistical precision necessary to deliver these products to global trial sites. Suppliers should know the requirements governing import and export, as well as the special handling criteria necessary for each type of delivery (i.e. cold-chain requirements, controlled drugs laws, complex/small volume distribution, etc.).
Unfortunately, a short lead time may also preclude the ability to source directly from the innovator/manufacturer of the commercial drug – a route that generally requires a bit more time. That said, this direct route often mitigates procurement risks through demand planning and development of long-term supply agreements, assuring stock for the duration of the trial, consistent pricing, requested documentation, and predictable, manageable timelines.
Leveraging Connections
Probably one of the most effective ways in which the risk can be taken out of the sourcing process, assuring commercial drug availability throughout the course of the trial, is to source directly from the commercial drug’s manufacturer – referred to as direct sourcing (indirect sourcing will also be discussed). Direct sourcing often requires basic trial detail disclosure to the innovator for supply approval; thus, pursuing this direct route can be arduous since trial sponsors may wish to maintain anonymity during the sourcing process – especially at the start of the trial. It should also be noted that the innovator may have no interest in making its drug available to a trial sponsor – especially if it’s for a head-to-head comparator seeking to prove superiority over its compound, or for biosimilar development. Global manufacturers may also not be well setup to handle “commercial” requests which do not fit their demand planning models. These scenarios make working with a sourcing specialist like Client-Pharma especially advantageous – saving time, resources and money!
Trial sponsors can leverage this direct sourcing route, while often preserving their anonymity, through partnership with a sourcing specialist who has cultivated robust relationships with innovators, manufacturers and distributors who can negotiate supply requirements on the sponsors’ behalf. Working with a specialist, like Client-Pharma who has such strong and extensive connections to drug manufacturers also brings numerous benefits beyond confidentiality.
A sourcing specialist can liaise directly with innovators/manufacturers to schedule production runs to meet both short- and long-term commercial drug requirements for one’s trial. This demand planning helps ensure reliability and assurance of supply for the duration of a trial, with the same quality of material throughout, while helping to maintain the flexibility of supply. This can be especially useful should supply requirements change due to a study being extended, if countries are added or if there are fluctuations or delays in anticipated patient enrollment.
In addition to effective demand planning, sourcing directly from a manufacturer enables access to larger, single lots with maximum shelf-life, and requesting specific batches when possible. Securing commercial drugs with a lengthy shelf-life provides adequate time for characterization, demonstration of bioequivalence, relabeling and repackaging (if needed), dosing patients over a specific timeline, all while minimizing the frequency of costly resupply.
Securing the proper paper trail for the commercial drugs (comparators, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries, consumables, etc.) can reduce exposure to risk and this is much simpler and easier to accomplish when dealing with the manufacturer directly. The manufacturer can routinely provide full pedigree documentation reflecting the chain of custody from the source to the designated point of delivery, along with full product documentation including Certificates of Analysis (CofA), Certificates of Conformity (CofC), Summary of Product Characteristics (SPC), Package Inserts (PIs), Batch Release Certificates (BRC), Statements of Authenticity (SofA), GMP compliance, Statements of Equivalency, Material Safety Data Sheets (MSDS), TSE/BSE documentation, and stability data (in case of any temperature excursions during the trial).
The availability of these documents is not guaranteed and often unavailable when sourcing commercial products via wholesalers, placing a sponsor at risk in terms of meeting regulatory requirements. While a wholesaler may be able to offer the lowest upfront product cost, the lack of robust relationships with innovators and manufacturers along with often having supply quota restrictions could result in an unreliable supply route throughout the course of a trial, ultimately leading to a much higher total acquisition cost in the end.
Although sourcing direct from innovators/manufacturers provides a multitude of benefits, there are also many reasons as to why a sourcing specialist like Client-Pharma would recommend not going direct; but instead, utilizing its robust network of audited and approved suppliers to obtain material indirectly – without contacting the manufacturer. A sponsor may want to maintain its anonymity, not disclosing any trial specifics, keeping all details confidential. Or another scenario could be due to condensed timelines, perhaps immediate supply is required at short notice, and sourcing indirectly – from the market – is the only way to secure material quickly with minimal or expedited lead times. Or in parallel, while the sourcing specialist works with the innovator to obtain material directly, sometimes up against long lead times that may be standard for certain products and manufacturing schedules, and possibly while the parties are finalizing supply agreements with the manufacturer, it is necessary to source indirectly – from a network of audited and approved suppliers in order to initially “seed” sites with essential material – while subsequent bulk supply orders are being incorporated into the manufacturer’s demand plan. And, there are situations where a manufacturer simply will not supply product; thus, procuring material from the market is the only option available.
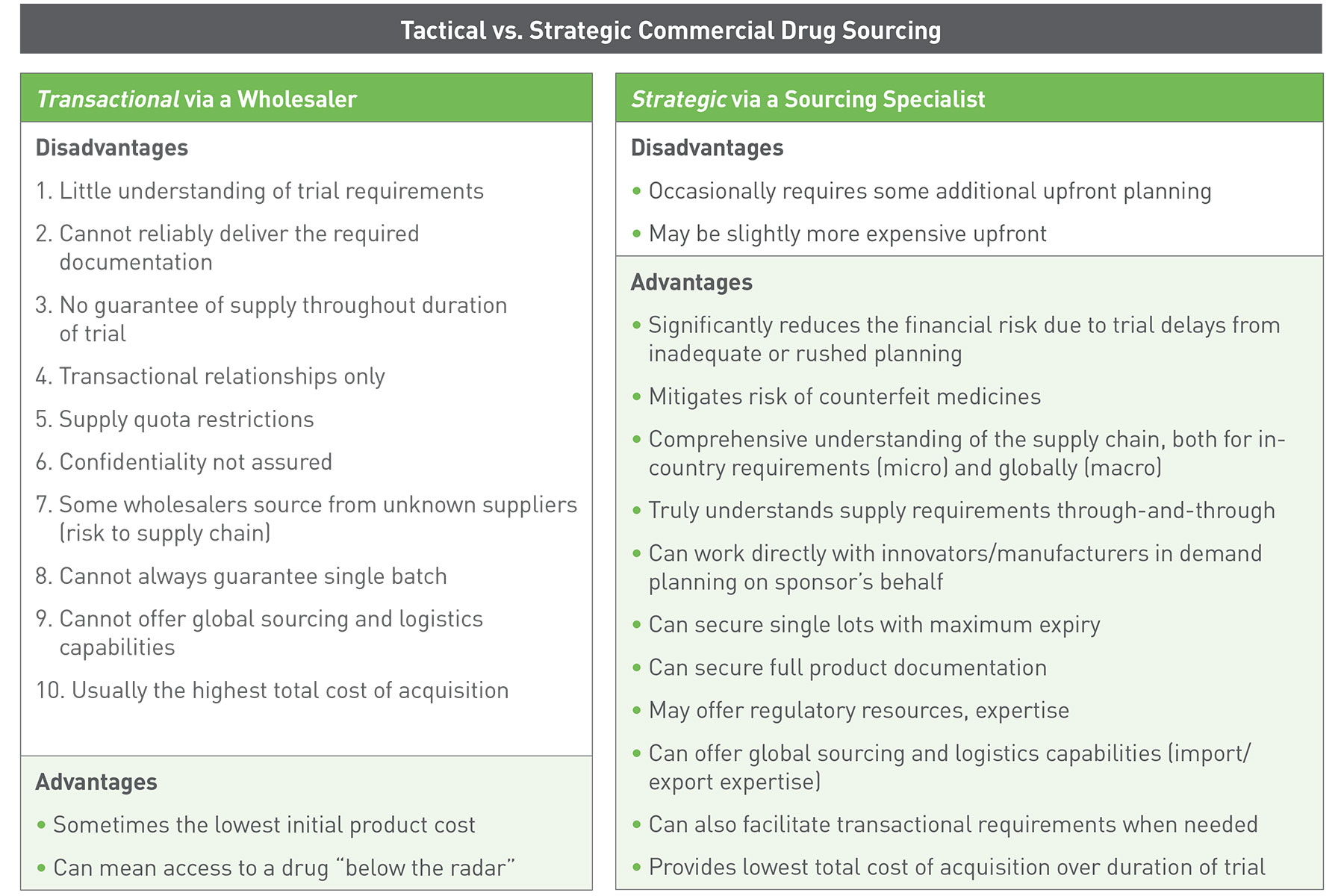
The table below compares the process of sourcing commercial drug supply via a wholesaler with that of sourcing clinical supplies via a sourcing specialist. While the initial product cost may be lower from a wholesaler, a number of disadvantages can lead to significant costs during the course of the trial. Utilizing a sourcing specialist like Client-Pharma minimizes the numerous risks of unexpected costs.
A Head Start
Probably one of the simplest ways to reduce risk during the commercial drug sourcing process is to start planning as early as possible, typically during the protocol design phase if feasible. A head start on planning is imperative, as commercial drug supplies are likely to be available from a wide variety of sources (direct from manufacturer or indirect from the market) across a number of geographies. Each source, however, may exhibit regional variations and will be governed by different trade regulations, all of which must be considered prior to selecting the most appropriate source.
Advance planning allows time to:
- Put a drug procurement strategy in place that allows assessment of all options and availability
- Evaluate regional variations of drug options, including dosage form, dosage strengths, API concentrations, visual and packaging presentations, etc.
- Review sourcing options in terms of regulatory requirements, reformulation, repackaging, and relabeling needs
- Establish required volumes and delivery schedules
- Evaluate pricing and total budget
- Confirm availability of required pedigree and product documentation
It’s All About Right Place, Right Time
 The ultimate goal of drug sourcing is to get the correct drug in the necessary quantities to the study site on time. Indeed, the efficient management and movement of clinical trial supplies, including all the commercial drug sourced, is an important factor in the success of any trial.
The ultimate goal of drug sourcing is to get the correct drug in the necessary quantities to the study site on time. Indeed, the efficient management and movement of clinical trial supplies, including all the commercial drug sourced, is an important factor in the success of any trial.
Making this process considerably challenging is the growing number and increasingly global nature of clinical trials. We know there has been important and meaningful progress in clinical development through the years and the number of registered global clinical trials has drastically increased. As of October 2019, there were nearly 319,000 registered global clinical programs, compared to only 2,119 registered global clinical trials back in 2000.1 Significant growth in the number and complexity of clinical trials conducted is happening in many regions around the world: China and the majority of Asia, Australia, Africa, Middle East, Eastern Europe, and South America (in addition to the more traditional regions of the past, North America and Europe).
An expanding number of trials and their geographic diversity place an added burden on both regulatory and supply/logistics teams to establish and maintain a compliant, efficient and secure supply chain across many borders. Requirements established by local regulatory agencies regarding the source of commercial drugs (i.e. whether a comparator sourced in the U.S. can be used in an ex-US-based trial) as well as general trade regulations governing import and export must be understood and followed. Further complicating supply and logistics are commercial drugs that need special handling, such as cold-chain or frozen requirements, controlled drugs, biological or genetic material, or complex/small volume distribution.
All of these factors are converging to put additional pressure on the supply chain and, if not managed effectively and efficiently, can result in delayed delivery of commercial supply to sites, which in turn, can lead to costly delays or interruptions in the trial. An agile, global inventory and logistics management process, supported by extensive regulatory expertise, is more critical now than ever to ensure compliance, supply chain security, prompt delivery of supplies, and global batch tracking and traceability.
Regulatory expertise specific to the countries to which the commercial drug(s) are being shipped also helps ensure that proper documentation accompanies supply. It is critical that trial sponsors remain updated on and attentive to the constantly evolving regulations affecting the geographies in which trials are running and maintain close relationships with key regulatory bodies should sourcing-related questions arise. It is also crucial having a partnership with a sourcing specialist, like Client-Pharma, who has the expertise to help navigate this complex landscape while guiding and managing – mitigating risks on all fronts.
Finding the Source
Once the requested commercial drug(s)/comparator(s) have been identified for a clinical trial, the task turns to locating a source for the drug. While the commercial product (comparator, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries, consumables, etc.) may be available from a variety of sources, regional variations in the drug itself make it important to have as broad a view as possible of available sources across many geographies.
Detailed knowledge of the global commercial drug sourcing landscape enables assessment of a range of options, leading to selection of the most appropriate, reliable and reputable source for a particular clinical trial. Caution must be exercised, however, when evaluating sources for your commercial drug supplies. Only those sources that are audited to ensure supply chain security and minimize the risk of counterfeit products should be considered.
Global sourcing capabilities also enable rapid identification of alternative sources for your commercial drug supplies should there be an unexpected change in production or the formulation of the originally sourced material (often more common with generic products). Immediate access to information on alternative sources helps minimize the possibility of delays in delivering commercial drugs to trial sites.
Single Sourcing
Many trial sponsors are interested in the concept of global single sourcing; that is, the supply of a commercial drug from a single geographic source to all trial sites globally. When considering single sourcing in support of a trial, it is important to weigh the pros and cons associated with this strategy.
Single sourcing offers a number of benefits to the trial sponsor, including:
- Management of one commercial drug source
- Reduction in possible local, in-country supply problems
- Consistency in data collected across all trial sites
- Greater flexibility (within regulatory guidelines) to shift supplies from one site to another based on enrollment and other parameters
Along with these benefits come a number of challenges. A trial sponsor should carefully evaluate single sourcing options from a regulatory standpoint and understand what is required in terms of supporting documentation. The FDA, for example, provides no formal guidance on single sourcing, so it is best to consult your commercial drug sourcing specialist, which will have the capabilities to advise you on a case-by-case basis and be in direct contact with agency officials on your behalf. Client-Pharma works with numerous competent authorities (FDA, MHRA, Health Canada, TGA, CFDA, PMDA, etc.) to support its clients’ global programs.
While single sourcing can ease the demands on internal supply and logistics departments, the dependency upon one source for your commercial drug supply presents a different form of risk and requires stringent evaluation and verification of the supplier. A sourcing specialist like Client-Pharma can ensure that these risks are fully quantified and mitigated.
In Summary
A clinical trial can come to an abrupt halt if the commercial drug supply is not available throughout the duration of the study, affecting trial timelines and having a significant financial impact on the sponsor. Delays at the start of a trial due to the inability to locate a reliable, secure source for commercial drug(s) or lack of the required product documentation are just as costly.
A transactional approach to sourcing, where the focus is on finding the commercial drug (comparator, standard-of-care treatments, adjuvant therapies, rescue meds, ancillaries, etc.) as quickly and inexpensively as possible, can increase the likelihood of these setbacks prior to and during the course of a clinical trial. Commercial drugs that are sought via a “last minute” approach can open up a company to numerous regulatory, operational, and financial risks.
Commercial drug sourcing and supply should be considered as an important strategic element of the planning and preparation for all clinical trials. The goal for the Clinical Supply and Procurement teams should be to have early visibility of commercial drug needs — timing, volume and geographic requirements — and the time needed to properly evaluate all sourcing options. Close communication between the sponsors’ clinical trial supply teams and its sourcing specialist partner enables advanced planning in all areas of clinical trial procurement and allows for identification of sourcing strategies that will minimize a company’s exposure to risks and possible significant, unexpected costs.
_
Andrea Chopek is President, North America at Client-Pharma, Inc., a specialty services company focused on worldwide commercial drug sourcing and supply for global clinical research and development. She can be reached at andrea.chopek@client-pharma.com
References
- Matej Mikulic. Total Number of Registered Clinical Studies Worldwide Since 2000. Statista. October 10, 2019.